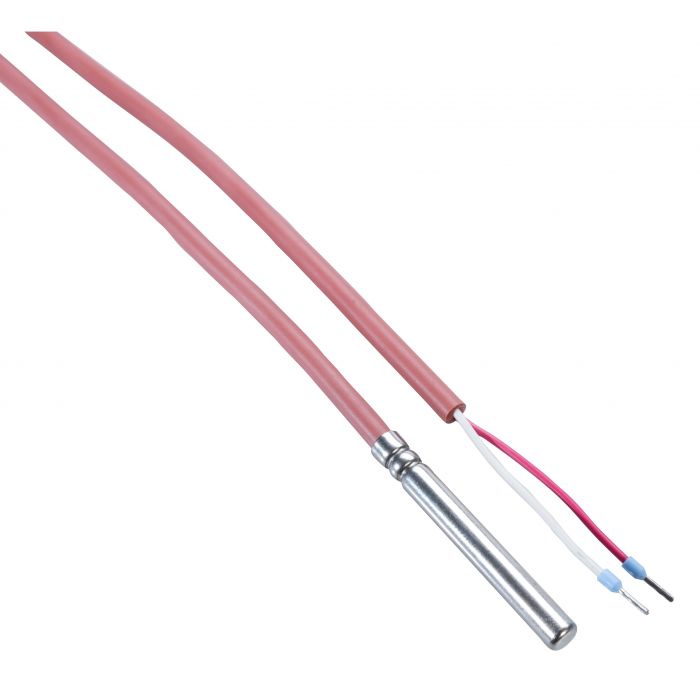
A pharmacy, chemist’s or drugstore is a physical location that, amongst others, distributes medication for patients. The distribution is subject to pharmacy legislation and pharmacies fall under the Good Distribution Practice (GDP) quality guidelines that are developed by the regulatory authorities and are designed to cover the product quality over the complete life cycle of the product.